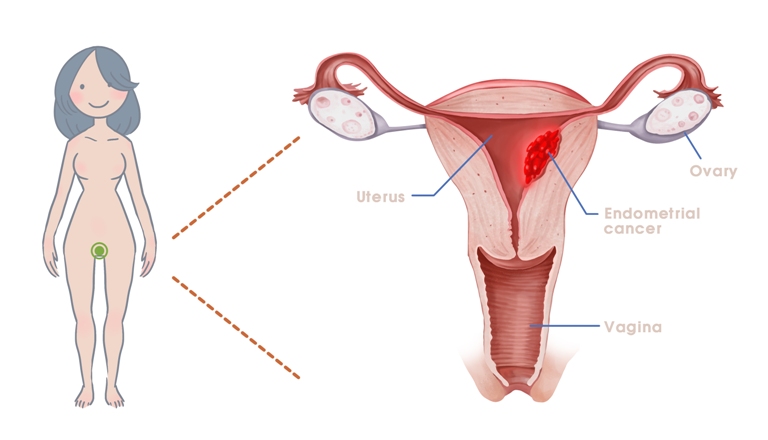
Endometrial cancer is a type of cancer that is usually found in the endometrial lining of the womb, which is also one of the most common cancers among women. Incidences rates have been tremendously growing in good economy regions such as North America, Europe, and other western societies; but more recent cases has proven to be seen within the Asia-Pacific region as well. Thus, According to American Cancer Society's official website indicates the majority of endometrial cancer patients are women over 40 years old, but due to various environmental factors (such as urban city environment, stress, obesity, and western diet influences just to name a few), women below the age of 40 years old has become the potential group of targets as well. Endometrial cancer is usually associated with high blood, pressure levels, excess of estrogen exposure and diabetes, there is no certain test for detection at the moment; the screening process involves several diagnostic tests followed up by confirmatory tests.
Thus, Guzip Biomarkers Corporation, a Taiwan medical startup set to revolutionize the diagnostic tool of endometrial cancer from 2018. Guzip Biomarkers Corp is a 2018 medical spinoff startup from Taipei Medical University, the company has dedicated itself into developing a diagnostic tool for endometrial cancer by utilizing “in vitro diagnostic (IVD)”as its main axis of the product development, which provides a smart and painless diagnosis plan for all the women across the global platform. According to co-founder Dr. Polly Lin as she stated during the interview: Guzip Biomarkers Corp’s vision is to be a medical company that dedicates to women’s health, by providing accurate, affordable, and safe medical alternative solutions to diagnose endometrial cancer. Guzip Biomarkers Corp’s core technology originates from an expert team led by co-founder M.D. Hung-Cheng Lai of Taipei Medical University in Taiwan. The product, MPap, is a real-time polymerase chain reaction (PCR) assay for methylation status of the several genes using the genomic DNA extracted from a cervical bush/swab. The kit serves as an assistance tool in endometrial cancer screening by combining traditional pap smears and methylation detection technology. The tests results can be utilized as a reference who is suffering from abnormal bleeding is susceptible to endometrial cancer, and allow doctors decide early on whether to undergo further evaluations such as invasive endometrial sampling and continue with other medical treatments.
During the interview, when discussing about Guzip Biomarkers Corp’s directions and its commercial strategies with Dr. Lin; she stated that they currently have two business models, LDT for service and IVD for products. In which by 2020 Q2, they are going to launch LDT service through a partnership with TSH Biopharm Corporation Ltd. Regarding IVD mode, the company preparing for a “pivotal” clinical performance study and expecting to receive the TFDA approval and release its IVD product by 2022. As it was shown in Guzip Biomarkers Corp’s previous studies, few biomarkers were validated in clinical samples, and the data showed that it’s high sensitivity and specificity. Due to the specimens (approx. 700 samples) are all collected here in Taiwan, Guzip Biomarkers Corp. would like to seek international cooperation to test its biomarkers in U.S. population and to evaluate the racial differences.
During our further discussions in terms of Guzip Biomarkers Corp’s goal for international cooperation, Dr. Lin also illustrated out a few challenges they shall face further down the road. Despite having Taiwan being first place to demonstrate proof-of-concept (POC) or commercial feasibility, it is undeniable that the United States remains the largest medical device market across the global platform; itself is an attractive market to have business cooperation with. The endometrial cancer diagnostic test includes biopsy procedures such as endometrial biopsy, hysteroscopy and dilation & curettage. Gold standard for endometrial cancer diagnosis is either endometrial biopsy or dilation & curettage combined with hysteroscopy. In the U.S. the confirmatory are comparatively expensive. For example, an average hysteroscopy can cost up to $4920 USD and endometrial biopsy can cost up to $473 USD. In which, Guzip Biomarkers Corp. will do its best to its ability and be the leading provider dedicated to the women’s healthcare arena, providing accurate, affordable, and safe medical solutions to diagnose endometrial cancer here in Taiwan and abroad.
InnoVEX 2020 Register Now!
If you have not yet registered for the InnoVEX 2020, make sure you complete your registration before the end of March to take advantage of the regular rate! Any registration as of April is subject to a 30% increase!